CNS Resources
The Digestive System of Vertebrates
Neuroendocrine control of the digestive system
Section Introduction:
The motor, secretory, digestive, and absorptive activities of the digestive system are coordinated by neuroendocrine control. Stimulation of the nervous system releases agents that produce rapid and transitory responses. The hormones released into the blood and paracrine agents that act on neighboring cells result in a slower and more prolonged response. The ingestion of food, reduction of its particle size, secretion of oral glands, initial stages of deglutition, and final stages of defecation are controlled principally by the nervous system, but most other functions of the digestive system are under both neuro and endocrine control. The large number of neurotransmitters, neuromodulators, hormones, and paracrine agents involved, and the fact that some of these agents are secreted by both neurons and endocrine cells complicates this distinction. Species comparisons are further complicated by differences in their sites of secretion and the receptors that respond to these agents. Neuroendocrine regulation of the mammalian digestive system is reviewed in Physiology of the Gastrointestinal Tract, 3rd edition, Volume 1, Section 1 (ed. Johnson et al. 1994). The following discussion concentrates on the major variations seen in the different classes of vertebrates (Stevens and Hume 1995).
Mammals - Neural control:
The mammalian digestive system is innervated by extrinsic and intrinsic neurons. The intrinsic neurons of the digestive tract are found in the enteric nervous system (ENS), which consists of afferent and efferent neurons with cell bodies in the myenteric and submucosal plexuses of the gut wall (Fig. 11.1). Receptors of the afferent neurons sense changes in the chemical composition, pH, or osmolality of gut contents, or the degree of gut wall distention, and transmit this information to other neurons in the plexus. Some of this information is transmitted to the central nervous system via extrinsic neurons, but most of it is transmitted to other enteric neurons that innervate the effector (muscle, secretory, or absorptive) cells of the digestive tract. Thus, the activities of the digestive tract are monitored and controlled largely by the neurons of the ENS. However, the responses of the ENS can be modified by the visceral efferent (autonomic) nervous system.
<img alt="Diagram of the wall of the small intestine" src="../images/dsv/GITFigures/NeurohumerolXSectionWallSmallIntestine%20F11_02.gif">Figure 11.1. Cross-sectional and cut-away diagram of the wall of the small intestine showing the extrinsic parasympathetic (a) and sympathetic (b) nerve fibers entering via the mesentery, and the submucosal (e) and myenteric (g) plexuses of the enteric nervous system. Cross section shows the successive layers of mucosa (c), submucosa (d), circular muscle (f), longitudinal muscle (h) and serosa (i). (Modified from Gershon and Erde 1980)
The extrinsic innervation of the mammalian digestive system consist of the parasympathetic and sympathetic neurons that innervate the digestive tract (Fig. 11.2) and its ancillary organs. The parasympathetic nerve supply is referred to as the cranial-sacral division, because its cell bodies are located in the brain and sacral segment of the spinal cord. Cranial nerves innervate the salivary glands and cranial esophagus, and join the vagus nerve supply to the pancreas, gall bladder, and ENS of the distal esophagus, stomach, midgut, and proximal hindgut. Axions with cell bodies in the sacral division of the spinal cord travel in the pelvic nerves and innervate the ENS of the distal hindgut. The cell bodies of preganglionic sympathetic neurons are located in the thoracolumbar segment of the spinal cord and synapse with neurons in paravertebral, cervical, celiac, or mesenteric ganglia, whose post-ganglionic axons innervate cells of the ENS.
<img alt="Visceral efferent innervationof the mammalian gastrointestinal tract" src="../images/dsv/GITFigures/NeurohumerolVisceralEfferentInnervationMammalianGIT%20F11_01.gif">Figure 11.2. Visceral efferent (autonomic) innervation of the mammalian gastrointestinal tract. The sympathetic enervation is shown to the left of the figure and the parasympathetic enervation is shown on the right. SCG; Superior cervical ganglion; CG, celiac ganglion; SMG, superior mesenteric ganglion; IMG, inferior mesenteric ganglion; IMN, intermesenteric nerve; LCN, lumbar colonic nerves; HN, hypogastric nerves; X, vagus dorsal motor nucleus and vagus nerve; PN, pelvic nerves; IAS, internal anal sphincter. (From Roman and Gonella 1981).
The terminal axons of parasympathetic neurons and preganglionic sympathetic neurons release acetylcholine, which is excitatory to neurons or effector cells. Therefore, they are referred to as cholinergic neurons. The terminal axons of the post-ganglionic neurons of the sympathetic nervous system release norepinephrine, which is generally an inhibitory agent, and are referred to as adrenergic neurons. The inhibitory effect of the parasympathetic neurons on effector cells appears to be due mainly to the inhibition of acetylcholine release from cholinergic neurons. However, stimulation of the extrinsic parasympathetic nerves of the gastrointestinal tract can result in cholinergic excitation, noncholinergic excitation, or nonadrenergic inhibition of effector cells. This is due to the release of a large variety of purine, amine, peptide, and other neurotransmitters or neuromodulators by neurones in the enteric nervous system (Table 11.1a,b).
Table 11.1a.
<img alt="Neurotransmitting and neuromodulating agents of mammals" src="../images/dsv/Tables/NeurohumerolTransmittingModulatingAgentsA%20T11_01a.gif">Abbreviations: 5-HT (5-hydroxytryptamine); AP, ADP, AMP (adenine and adenine nucleotides). (from Burks 1994 and Dockray 1994)
Table 11.1b.
<img alt="Neurotransmitting and neuromodulating agents of mammals" src="../images/dsv/Tables/NeurohumerolTransmittingModulatingAgentsB%20T11_01b.gif">Abbreviations: VIP (vasoactive intestinal peptide); GRP (gastrin-releasing peptide). (from Burks 1994 and Dockray 1994)
Mammals - Endocrine control:
Hormones and paracrine agents are secreted by endocrine cells in response to conditions in the digestive tract. Hormones are secreted into the blood and act on distant target organs. Paracrine agents act on neighboring cells. Some of these agents are produced by both endocrine and nerve cells and can act as hormones, paracrine agents, neurotransmitters, or neuromodulators. The principal gastrointestinal hormones and paracrine agents and their effects on the digestive system of mammals are listed in Tables 11.2a and b. The sites and stimuli for release of the hormones gastrin, gastrointestinal peptide (GIP), secretin, and cholecystokinin are shown in Figure 11.3.
Table 11.2a.
<img alt="Effects of hormones and paracrine agents on the digestive system of mammals (part 1)" src="../images/dsv/Tables/NeurohumerolHormonesParacrineAgentsA%20T11_02a.gif">(from Walsh 1994)
Table 11.2b.
<img alt="Effects of hormones and paracrine agents on the digestive system of mammals (part 2)" src="../images/dsv/Tables/NeurohumerolHormonesParacrineAgentsB%20T11_02b.gif">(from Walsh 1994)
<img alt="The role of hormones in controlling gastric acid secretion, pancreatic secretion of salts and enzymes, and contraction of the gall bladder" src="../images/dsv/GITFigures/NeurohumerolRoleHormonesControlling%20F11_03.gif">Figure 11.3. The role of hormones in controlling gastric acid secretion, pancreatic secretion of salts and enzymes, and contraction of the gallbladder. Release of gastrin, from the pylorus, initiates the secretion of hydrochloric acid by the oxyntic cells in the fundus. The duodenal-jejunal hormones secretin and cholecystokinin-pancreozymin (now referred to as simply cholecystokinin or CCK), initiate the secretion of, pancreatic fluids and enzymes, respectively. Gastric-inhibitory peptide (GIP) inhibits gastric acid secretion. Open arrows indicate inhibition. Closed arrows indicate stimulation. (From Bentley 1982).
Enteroglucagons, peptide YY, somatostatin, motilin, neurotensin, and melatonin have been isolated from intestinal mucosal cells. Melatonin was also isolated from esophageal and gastric mucosa. Pancreatic polypeptide is present in pancreatic islet and F cells. Some hormones that act on the digestive system are secreted by other organs of the body. For example, a decrease in plasma volume stimulates the release of the hormones renin and aldosterone from the kidney. Renin generates the release of angiotensin, which reduces urinary excretion, stimulates thirst, and the gradual release of more aldosterone. Aldosterone stimulates the absorption of Na+ and, therefore, water by the kidney, salivary ducts, and hindgut.
Other vertebrates:
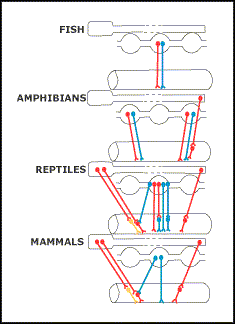
Burnstock (1969) concluded that there are some marked differences in the autonomic nervous system of different classes of vertebrates (Figs. 11.4 and 11.5). Vagal innervation of the digestive tract does not extend beyond the stomach of fish. Vagal stimulation produced a nonadrenergic, noncholinergic, inhibitory response, whereas stimulation of spinal autonomic nerves produced both adrenergic inhibitory and cholinergic excitatory responses. Autonomic innervation of the adult amphibian gastrointestinal tract appeared similar to that of fish, except for evidence of a sacral cholinergic nerve supply. However, the autonomic nervous system of reptiles appeared similar to that of mammals, with a complete exchange of cholinergic excitatory function from sympathetic to parasympathetic outflow, retention of some vagal nonadrenergic, noncholinergic, inhibitory innervation, and the sacral parasympathetic innervation of the hindgut. The autonomic nervous system of birds appeared to be similar to that of mammals.
Figure 11.4. Diagrammatic representation of the autonomic cholinergic excitatory (red line), adrenergic (yellow line) and nonadrenergic inhibitory (blue line) nerves to the stomach of vertebrates. (From Burnstock 1969).
Figure 11.5. Diagrammatic representation of the autonomic cholinergic excitatory (red line), adrenergic (yellow line) and nonadrenergic inhibitory (blue line) nerves to the intestine of vertebrates. (From Burnstock 1969).
Although most of the agents that serve as neurotransmitters, neuromodulators, hormones, or paracrine agents in mammals are present in other classes of vertebrates, they can different in their site of origin, function, or target (Stevens and Hume 1995). Most of the agents identified as neurotransmitters or neuromodulators in mammals have been found in the nervous system of other classes of vertebrate, by either direct measurement or demonstration of a similar immunoreactivity. Acetylcholine, norepinephrine, and immunoreactivities to substance P and bombesin were demonstrated in the nervous system of birds, reptiles, amphibians, and fish. Gastrin-, CCK-, secretin-, pancreatic polypeptide-, somatostatin-, and neurotensin-like immunoreactivity have been found in all classes of vertebrates. Table 11.3 lists the agents found in the gut of chickens, and those found in caiman and frogs are listed in Table 11.4 and Figure 11.6.
Table 11.3.
<img alt="Immunoreactive cells in the GIT of chickens" src="../images/dsv/Tables/NeurohumerolEndocrineCellsChicken%20T11_04.gif">Abbreviations: SOM, somatostatin; APP, avian pancreatic polypeptide; PYY, polypeptide YY; GLUC, glucagon; SEC, secretin; VIP, vasoactive intestinal peptide; GAS, gastrin; CCK, cholecystokinin; NT, neurotensin; BN, bombesin; SP, substance P; ENK, leu-enkephaline; MOT, motilin; 5-HT, serotonin. PYY data (El-Salhy et al. 1982, recently hatched chicks), ENK data (Alumnets et al. 1978, chickens), 5-HT data (unpublished observations, chicks at hatching), CCK duodenum data (Larrson and Rehfeld 1977, chickens), All other data (Rawdon and Andrew 1981, chicks at hatching). (from Rawdon 1984)
Table 11.4.
<img alt="Gastrointestinal endocrine cells in the caiman" src="../images/dsv/Tables/NeurohumerolEndocrineCellsCaiman%20T11_03.gif">—absent, + rare (not detected in every animal), + few (detected in every animal but not every section), ++ moderate, +++numerous. (from Yamada et al. 1987)
<img alt="Populations of endocrine cells that are immunoreactive to peptides in the digestive system of the frog" src="../images/dsv/GITFigures/NeurohumerolEndocrineCellsFrog%20F11_06.gif">Figure 11.6. Populations of endocrine cells that are immunoreactive to peptides in the digestive system of the frog Rana catesbeiana. (From Fujita et al. 1981).
The chemical structures of gastrin and CCK can vary and some of these agents appear to act on different receptors in different classes of vertebrates (Vigna 1983, 1986). Six different gastrin-like molecules have been isolated from some species and many amino acid substitutions are found in different species. the structure of CCK also varies with species. However, its hormone activity resides in the C-terminal octapeptide, and the C-terminal pentapeptide of CCK is identical to that of gastrin. Many of the 27 amino acids in secretin are also shared with GIP, glucagon, and VIP. Therefore, the gastrin-CCK and secretin families of hormones appear to have evolved from different ancestral peptides (see next section: 12. Evolution of the Digestive System).
Next section: Evolution of the digestive system